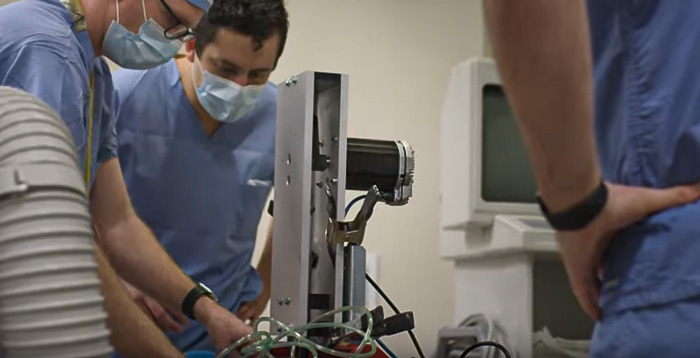
In the midst of the COVID-19 pandemic, medical teams across the country are experiencing critical shortages. As hospitals run out of available ventilators, doctors are turning to anesthesia workstations, BiPAP machines, and CPAP machines to help ventilate patients. As doctors exhaust their supplies of ventilators and quasi-ventilators, they are scrambling to find alternatives.
At first, the solution may seem obvious—produce more ventilators—but by the time manufacturers are able to scale up production to meet current and future demand, it will be too late.
Given that scaling up conventional ventilators will take too long, medical professionals need additional solutions. At some point, the only remaining option will be to manually ventilate patients with Ambu® bags and hope that conventional ventilators become available. Although Ambu bags are readily available, there are not enough trained clinicians (nurses, doctors, respiratory therapists, etc.) to operate these devices for every patient in need.
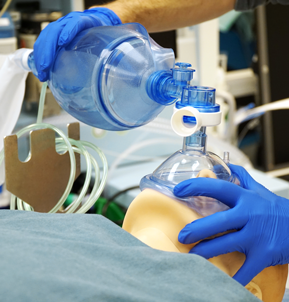
Operating an Ambu bag requires a clinician to actuate the bag about 10-30 times per minute without stopping—an action that quickly becomes tiring. When one clinician fatigues, a new one must take over, and this cycle must continue until the patient recovers or a ventilator becomes available.
Over the last few weeks, Teknic has been working on a project that automates the operation of Ambu bags (i.e. clinicians no longer need to actuate the bag—which frees up valuable resources for other tasks). Below, we delve into the details of the project.
Project Overview
In mid-March, a team including Dr. Stephen Richardson (an anesthesiologist at the University of Minnesota), Jim McGurran (an engineer from MGC Diagnostics), and a small group from the Earl E. Bakken Medical Devices Center conceived the idea of a one-armed robot (more technically, a single-axis linear actuator) to automate human ventilation using an Ambu bag.
When the team had an early working prototype, they contacted Teknic (on a recommendation from Digi-Key, a large electronics distributor) for advice on the project’s motion control requirements. In just over two weeks, our collective team brought the device all the way through concept, prototype, and production.
The machine, internally nicknamed “Ambu-bot” by Teknic, was not designed to replace ventilators. Rather, it was designed to automate the manual ventilation typically performed by medical personnel so that clinicians in over-stressed hospitals can treat other sick patients.

We left out sophisticated adjustments and sensors commonly found (and required) in conventional ventilators in order to drastically speed up the design, prototyping, testing, and production to meet the urgent need.
While using this machine, clinicians will be required to monitor patients more closely than patients on a conventional ventilator. However, this device allows a single clinician to simultaneously monitor multiple patients, opposed to one clinician manually ventilating one patient.
The machine does all of the required manual labor consistently and continuously, and a clinician can monitor multiple patients at the same time to ensure that each patient’s vitals (e.g., blood gases) stay in an acceptable range.
Below is a more detailed timeline of the project.
Conception
Dr. Steve Richardson and Jim McGurran started the design. They used a motor they had on hand and completed the first prototype in one day. That motor was powerful enough to spin the actuator and move the piston, but it was unable to compress the Ambu-bag. They reached out to Teknic (on a recommendation from Digi-Key) for advice on the project’s motion control requirements.
Day 1: Initial Prototype Design
Teknic and the team iterated on the existing design. We updated solid models and created a number of conceptual examples. The team set a target to have a functioning prototype within two days and created a list of goals for the actuator. It needed to:
- Be reliable and capable of running 24/7
- Have adjustable speed control
- Have adjustable compression (stroke volume)
- Be easy to build and have an open-source design
- Be ready for scaled up production (1,000 per week) in two weeks, and mass production (10,000 per week) within a few weeks after that
- Be simple in concept and universally applicable
- Be easily used by medical personnel with minimal training time
It was important for the prototype to work on the very first try. We needed to collect critical test data (electro-mechanical and clinical) and continue to iterate on the design while we incorporated that test data. A failure at this stage would have been a major roadblock on the critical path of the project.
Below is an early prototype of the actuator using some easy-to-manufacture (i.e. easily machined or 3D printed) components to actuate the bag.
Day 2: First Prototype Trial
Engineers worked around the clock, and within 24 hours, we had a few different prototypes up and running. The prototype featured in the video below was not pretty (we used a 4×4, some plywood, and some PVC pipe), but it proved to us that we were on the right path.
You may be wondering why we chose not to incorporate more advanced features (e.g., variable positioning, back and forth motion ![]() ) in this application. When the University medical staff contacted Teknic, we suggested that the team should exclude any proprietary ClearPath features from the actuator design, or any features available only on position control motors (e.g., other servo and stepper motors). We suggested this to ensure that a wide variety of motors could be used in the design, especially motors that are easily sourced in high volumes, such as windshield wiper motors.
) in this application. When the University medical staff contacted Teknic, we suggested that the team should exclude any proprietary ClearPath features from the actuator design, or any features available only on position control motors (e.g., other servo and stepper motors). We suggested this to ensure that a wide variety of motors could be used in the design, especially motors that are easily sourced in high volumes, such as windshield wiper motors.
Although we would have loved to incorporate more sophisticated features into the ventilator, the added complexity would have slowed down the project due to the extra time required to design, test, document, etc. These additional features also would have compromised manufacturers’ ability to source components.
We ruled out features such as reverse direction capability (at variable cam angles), custom speed vs. angle (e.g., to change the i/e ratio), dwells or pauses in motion, etc. Remember, a critical goal of this project was to move from concept to large-scale development in an extremely short window.
Day 4: Functional Test at the University of Minnesota
We iterated on the design multiple times in rapid succession and the doctors at the University began laboratory testing. The video below provides some of the product details and gives a brief overview of the testing process.
A question we’ve been asked is, “Why did [the team] start testing with a ClearPath motor if you planned to use wiper motors in high volume (tens of thousands per week)?”
We decided to use Teknic’s ClearPath motors on the initial prototype units for a number of reasons. Because the team asked Teknic to provide the motor and controls expertise, it made sense for us to start with what we had immediate access to.
The ClearPath motors were readily available in any configuration that made sense for the design, and we were confident that we would have the prototype actuators running quickly on the first try. A redesign at Day 4 would have delayed the critical task of collecting laboratory data and we didn’t have time for delays.
The team had a number of other reasons to use Teknic’s ClearPath motor in the initial prototype units:
- The model that we selected had enough power even in a direct-drive mechanism and at worst-case loading (i.e. we didn’t need to source gearing, even if it was beneficial for other reasons).
- The electronics (drive and controls) are integrated, so the prototype didn’t require any external circuitry.
- A servo-controlled motor eliminated any unforeseen issues related to speed variation under load.
- The motor could have been switched to bi-directional velocity mode or reciprocating positional mode in seconds, if necessary.
- The motor’s firmware reported operational data in real-time (e.g., torque vs. time, actual velocity, temperature, position, bus voltage)
- We could have limited the torque to an arbitrary value (for safety or to mock-up the feasibility of using smaller motors).
- We had over 1,000 variations in torque/speed characteristics to choose from. All of these combinations had identical electrical/software interfaces, and we could have shipped any variation immediately.
- We were able to pre-program the ClearPath-MC series and the application did not require any software during actual operation.
Day 8: An Update on the Project
We continued to iterate on the design and settled on a version that did not use any gearing at all. While this change required an increase in motor torque, it also meant that the eventual manufacturers of this device didn’t need to source or construct gearing. Even this relatively simple change was helpful to achieve our goals.
The team started building pre-production quantities and Teknic began working on a preliminary design for a new motor drive. This new drive used the same input as the ClearPath system, but drove a less sophisticated motor. This was to further simplify motor production in mass volume from a wide variety of sources.
Day 11: FDA EUA Submission and the Beginning of Manufacturing
Governor Doug Burgum of North Dakota placed an order for 2,000 units in anticipation of the FDA Emergency Use Authorization. Appareo Systems of North Dakota began sourcing components (including Teknic motors), manufacturing parts, and assembling the devices for rapid deployment.
Day 15: Shipping Began and Another Company Joined the Team
On Day 15, Teknic began shipping motors to Appareo Systems for assembly and production. Boston Scientific, a large medical devices manufacturer, officially announced that they would begin manufacturing these devices as well.

Day 20: Teknic Continued Shipping Motors for Production
Team members from the University of Minnesota and Boston Scientific met with the FDA. According to the team at Boston Scientific, “[The FDA group] greatly appreciates the fast response we are delivering with appropriate use of technical standards, quality and design controls, risk management processes and manufacturing practices to ensure device safety and performance.”
Day 28: The FDA Granted EUA Approval
The FDA formally granted Boston Scientific and the University of Minnesota an Emergency Use Authorization for the Ambu-bot under the official name “Coventor Automatic Adult Manual Resuscitator Compressor.” Boston Scientific announced plans to build 3,000 units, and then more as needed.
Prototype Design Files:*
- CAD files: Prototype Automated BVM Ventilator.zip
*Please note that these files are out of date as they were our first prototype version. Teknic and other parties are working hard on improvements and enhancements and we plan on posting updated files soon.
To Our Partners:
Teknic would like to give special thanks to all of the team members who participated on this project: